The Intriguing Link Between Entropy, Heat, and the Passage of Time
Written on
The interplay between entropy and heat is a nuanced one, significantly influencing the one-way progression of time. While popular science often oversimplifies entropy as mere 'disorder,' the deeper connection between entropy and heat is frequently overlooked. This essay aims to break down this intricate relationship into more understandable terms for those without a technical background.
Previously, in my discussion about the origins of entropy, I recounted how Rudolf Clausius and his contemporaries stumbled upon the concept while seeking to enhance the efficiency of steam engines. To grasp the delicate connection between entropy and heat, we will continue from that narrative.
For readers pressed for time, I will summarize the key elements regarding entropy's origins. However, for a thorough understanding, I highly encourage you to read the origins first. Without further delay, let's dive in.
Recap — The Birth of Entropy
In the mid-1800s, steam engines were at the forefront of the industrial revolution, enabling rapid and efficient transport of people and goods.
Nonetheless, these engines faced a major flaw: they wasted up to 95% of the heat generated from burning fuel (like coal or wood) as environmental waste, a staggering inefficiency that vexed many engineers and scientists.

Generations of researchers, including Lazare Carnot and his son Sadi Carnot, dedicated themselves to solving this inefficiency, which eventually led to the emergence of thermodynamics.
Lazare Carnot introduced the concept of work loss due to mechanical shocks and accelerations, while Sadi Carnot elaborated on thermodynamic reversibility. Rudolf Clausius expanded these ideas into the concept of irreversible heat loss, which he later named entropy.
Recap — The Connection Between Steam Engines and Entropy
In relation to steam engines, the principle can be stated as follows:
> The combustion process that powers the steam engine inevitably results in irreversible heat waste, leading to degradation.
This statement, while simplistic, is crucial for understanding the relationship between heat and entropy.
To further explore this, let's consider the question:
> Why is heat waste an unavoidable outcome in steam engines?
Understanding the Steam Engine
Steam engines operate by converting heat from burning fuel into steam, which expands to create pressure that moves a piston within a cylinder.
After the steam expands, it cools down (either through active or passive means), allowing the piston to return to its starting position, at which point fresh steam pushes it down again.
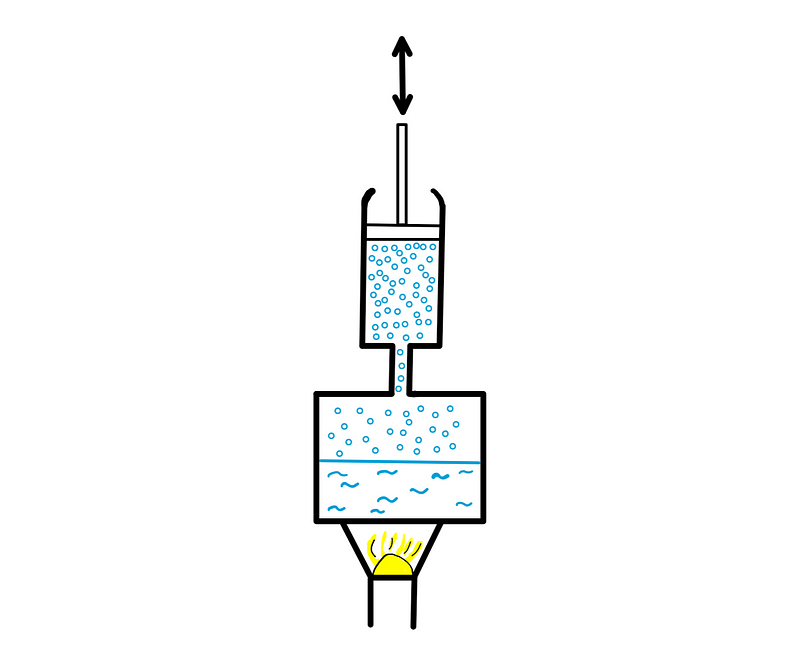
This process results in a linear to-and-fro motion of the piston, which was subsequently converted into rotational motion to drive the wheels of locomotives.
Now that we understand the operational mechanism of steam engines, we must answer: Why is heat waste an inescapable aspect of their operation? We still lack a critical piece of the puzzle.
A Childhood Memory
As a child, I was fascinated by the iron and its shape, despite my father's warnings against touching it, cautioning that I would eventually get burned.
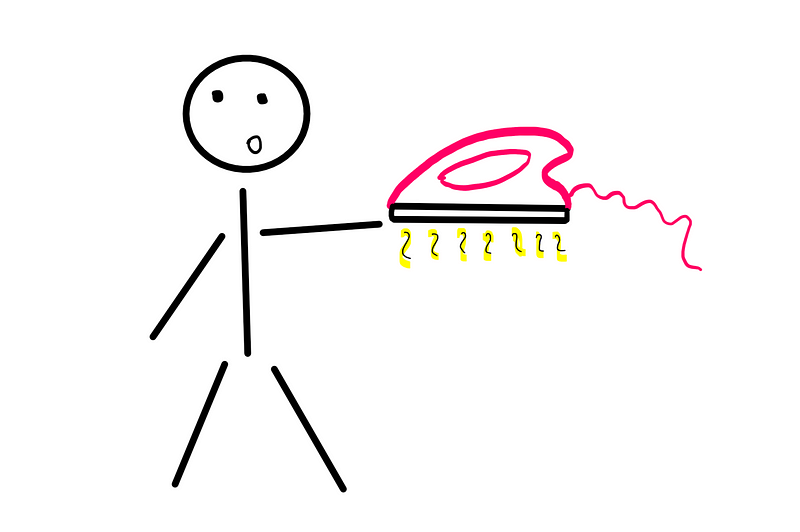
I eventually succumbed to curiosity and touched the hot iron, feeling a painful burn almost instantly.
Such experiences often require firsthand interaction for full comprehension; mere words fall short.
But why did my hand hurt? The hot iron raised the molecular speed of my hand's tissue, leading to the sensation of heat and pain.
The Essence of Heat
Heat can be defined as the energy associated with the average speed of molecules within an object. The iron's surface molecules were vibrating faster than those in my hand, and upon contact, the faster-moving iron molecules collided with the slower ones in my hand, increasing their speed.
This transfer of energy was perceived by my brain as heat. While earlier theories suggested an invisible "caloric" substance flowed from hot to cold bodies, today we understand it as the transfer of heat energy.
The Relationship Between Heat and Entropy
In the origins of entropy, I described how a high-entropy macrostate offers numerous possible micro-configurations, while a low-entropy state has fewer configurations.
When the iron is hot, its molecular motion increases, leading to higher entropy. Conversely, my cooler hand has lower entropy.
Upon contact, the faster iron molecules raised the speed of those in my hand, resulting in a rise in my hand's entropy.
The Intricate Link Between Heat and Entropy
Thus far, we’ve established that the hotter iron has higher entropy than my cooler hand. However, during this interaction, the temperature and entropy of the iron decreased slightly while those of my hand increased.
This indicates that for heat to flow from the iron to my hand, entropy must also be transferred. In essence, entropy flows with heat. This forms the crux of the relationship between heat and entropy.
With this understanding, we can tackle our initial inquiry:
> Why is heat waste an inescapable aspect of steam engines?
Understanding Heat Waste in Steam Engines
Recall that the steam engine’s piston returns to its original position after each cycle, necessitating a return of the entire engine's macrostate.
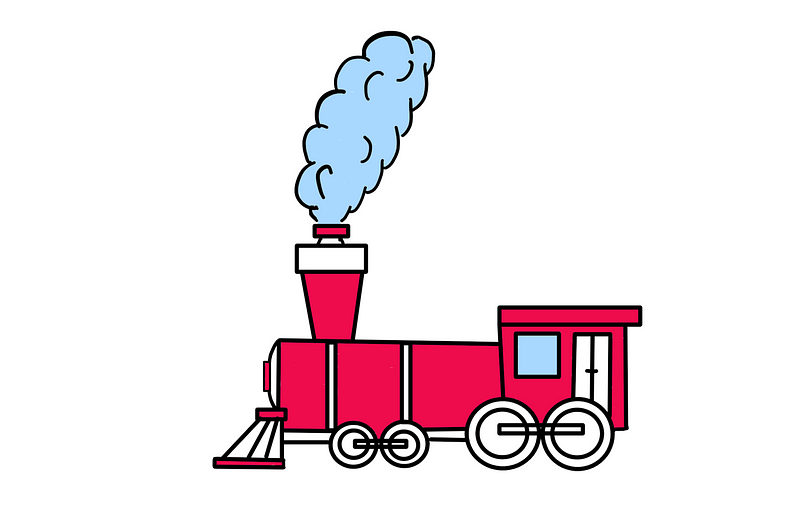
If the steam engine resets its macrostate at the end of each cycle, its entropy must also reset. As we noted, entropy is linked to heat flow.
Thus, if the steam engine resets its entropy at the end of each cycle, it must also release heat to the environment. This realization clarifies why heat is essential in steam engine operation, yet it does not fully explain the concept of 'heat waste.'
Consider this: the steam engine takes in heat from burning fuel but also releases it at the end of each cycle. Why, then, does it produce heat waste?
A Thought Experiment
To clarify this, imagine two identical heaters: one in a cold room and the other in a warm room, both producing the same amount of heat.
In the colder room, air molecules, having lower average molecular speed and entropy, start moving faster upon heating. Conversely, in the warmer room, the increase in molecular speed is minimal due to the higher initial entropy.
Thus, the takeaway is that the difference in temperature impacts entropy flow.
Now, returning to our steam engine, we realize the critical link: the temperature disparity between the burning fuel and the steam engine's surroundings.
This difference allows the steam engine to expel only a portion of the heat collected to release all the entropy acquired from the burning fuel. The remaining heat is used to drive the piston, referred to as useful heat, while the heat released to the environment is termed 'heat waste.'
The Paradox of Reducing Entropy
An intriguing aspect of steam engines is their ability to consistently reduce entropy at the end of each cycle, seemingly contradicting the second law of thermodynamics, which states:
> The entropy of an isolated system that is evolving spontaneously can never decrease.
So, how does the steam engine reduce its entropy? The answer lies in the definition of isolated systems.
To fully understand the second law's application, we need to consider the entire system, including the engine and all its interacting components, such as surrounding air.
When we account for the total entropy of the complete system, we find that while the steam engine's entropy decreases, the overall entropy of the entire system increases.
This mirrors the earlier example of the iron and my hand, where the iron's entropy dropped slightly as my hand's entropy rose.
Entropy and the Passage of Time
In previous discussions, I noted that entropy is central to the reason time flows in one direction, and reversibility is unattainable. This section will touch upon that concept.
Steam engines are not exempt from the second law; they naturally tend toward increasing entropy. However, humans have engineered them to counteract this tendency by transferring accumulated entropy to the surroundings, which necessitates releasing heat waste.
This principle applies broadly to all man-made devices facing the natural tendency to increase entropy. By locally reducing entropy, these devices maintain their structural integrity, albeit at the cost of inevitable heat waste.
To connect this to time flow, we can ask: If devices that locally reduce entropy contribute to a global increase in entropy, where does that entropy go? The answer is straightforward: it moves forward in time.
Final Thoughts
As you reflect on these captivating concepts presented in this essay, I want to reiterate a crucial point:
> All physical systems are likely to transition from lower to higher entropy states over time.
So, where does the ultimate limit lie? Answering that question will require a discussion of its own, which I intend to explore in future essays. For now, I hope you found this exploration enjoyable!
If you wish to support future content, consider contributing on Patreon.
Reference: Brian Greene.
You can read the original essay here.